Projects
Overview
Immune defense against intracellular pathogens and cancer cells depends on cytotoxic T lymphocytes. They are not alone and have evolved in the adaptive immune system to cooperate with myeloid cells and especially with dendritic cells, which are able to educate naive T lymphocytes. The T lymphocyte, initially naive and ignorant, once educated by the dendritic cell, becomes a powerful fighter capable of eliminating pathogen-infected or tumor cells. The communication between dendritic cells and T lymphocytes depends on two essential events:
- The presentation by the dendritic cells of antigenic peptides derived from pathogens or tumors, and
- The ability of the T cell to recognize these antigens and to modify its behavior according to the signals it receives from the dendritic cell.
Our lab is interested in the molecular mechanisms that:
- Support antigen presentation in dendritic cells.
- Allow T cell activation by pathogen- or tumor-derived antigens.
To study the molecular mechanisms involved in antigen presentation by dendritic cells and more broadly by myeloid cells, as well as T cell activation by antigen receptor-mediated signaling, we use in vitro methods such as in situ labeling and quantitative mass spectrometry, advanced microscopy, combined with in vivo experiments using murine models of anti-tumor immunity and vaccination.
Antigen Presentation by Dendritic Cells to T cells
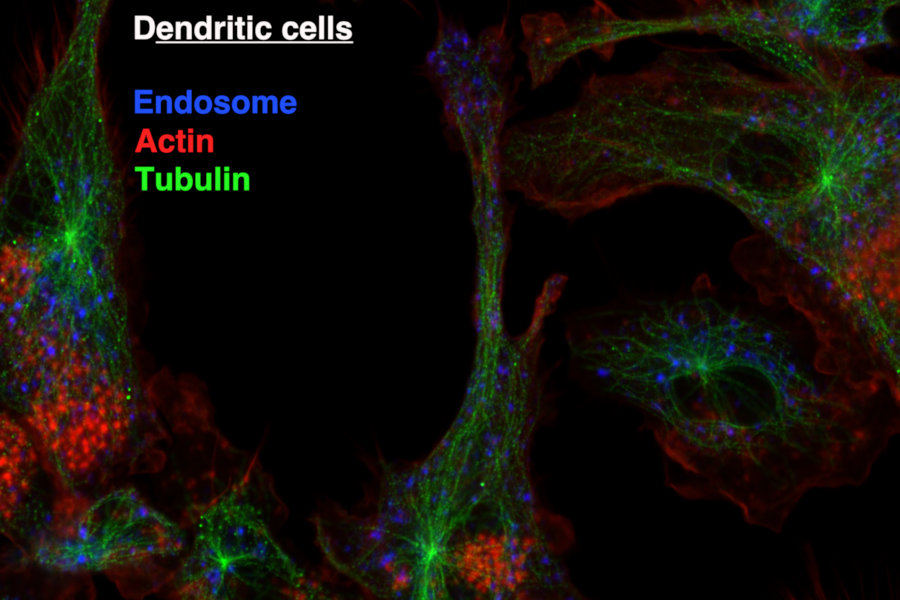
Dendritic cells take up infected cells or tumour cells, process them and generate antigenic peptides that are bound to major histocompatibility class I complex (MHC-I). The generation of antigenic peptide and their binding on MHC-I molecules involves complex molecular mechanisms in dendritic cells, in which endosomal trafficking has a major, but incompletely understood, role. We are investigating the signalling pathways that control the endocytic trafficking and subsequently antigen presentation abilities of dendritic cells.
Trafficking and signalling of Immunoglobulin receptors (FcRs)
FcRs family contains activating and inhibitory Fc immunoglobulin Receptors (FcRs) that control the immune homeostasis and inflammatory response, but also adaptive immune responses via MHC class I antigen cross-presentation. Using cellular and molecular biology assays, such as cell imaging or in situ biotinylation and quantitative mass-spectrometry, we investigate how intracellular trafficking modulates the activation of both activating and inhibitory FcRs. In addition, we use in vivo models of FcR-mediated diseases, such as arthritis and allergy, to understand if particular steps of FcR intracellular trafficking dictate the outcome of FcR ligation, such as maintenance of immune homeostasis, triggering of inflammatory response or initiation of adaptive immune responses.

T cell activation by antigen T cell Receptors (TCR) and Chimeric Antigen Receptors

To identify new players in the TCR signaling pathway, we have established the method of in situ protein biotinylation using the ascorbate peroxidase variant APEX2. In the presence of hydrogen peroxyde (for 30 seconds), APEX2 transforms biotin-phenol into highly reactive short-lived radicals (< 1ms) that diffuse at less than 20 nm and induce biotinylation of proteins in its proximity. The biotinylated proteins are purified, identified and quantified by mass spectrometry, allowing characterization of transient protein interactions with excellent temporal resolution. This method identified novel protein kinases associated with TCR signaling, half of which were not previously implicated in T cell activation. In addition, the in situ biotinylation assay we established can be applied to other scientific questions developed in our laboratory.
Modulation of macrophage function by autophagy
Macrophages (MF) mediate tumor cell killing via antibody-dependent cellular phagocytosis (ADCP) or cytotoxicity (ADCC), two phenomena that are strongly dependent on Fc IgG receptor (FcγR) function. The effector mechanisms of ADCC are complex and not fully understood, but are strongly dependent on the maturation of endocytic vesicles. Since autophagy is closely linked to the maturation of endocytic pathways, we are investigating the impact of autophagy modulation in myeloid cells on their ability to kill tumor cells. Based on our preliminary data, we propose to use autophagy to enhance the efficacy of chimeric antigen receptor macrophages (CAR-MF).

New transcription factors involved in neuro-inflammation
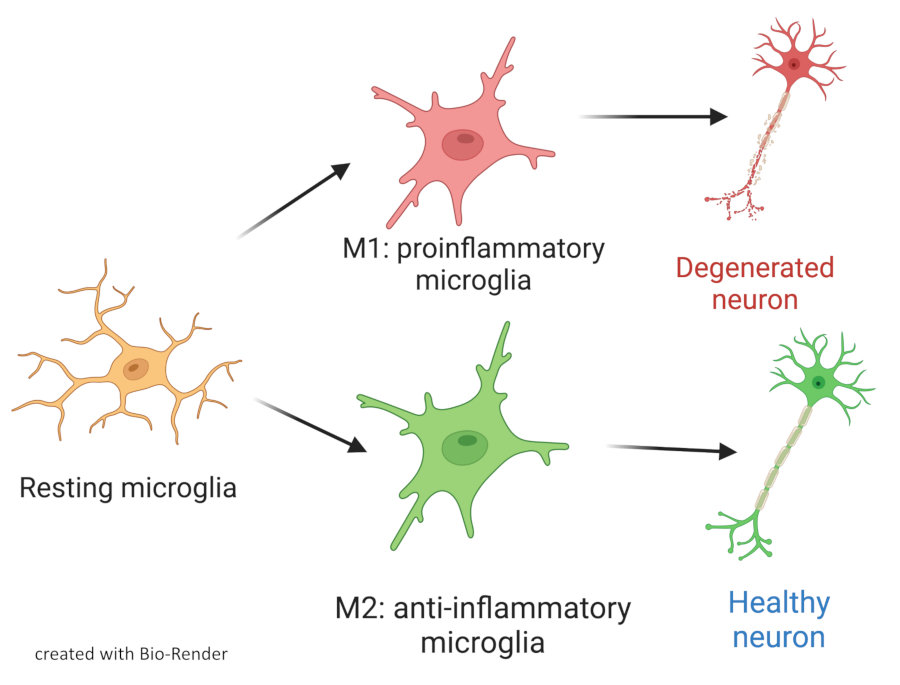
Inflammation is closely associated with diseases of the central nervous system (CNS). Microglia (MG) are macrophage-like cells resident in the brain and retina that are essential for normal CNS development and support tissue repair and remodeling. They are key players in neuroinflammation and neuroprotection by removing pathogens, cell debris and toxic molecules through phagocytosis, but can also regulate synaptic plasticity. We are investigating the role of a novel transcription factor in mouse models of neuroinflammation using transcriptomics, cell biology and in vivo models of multiple sclerosis and Alzheimer disease.